Free Clinical Research Coordinator Training Checklist

[Your Name]
[Your Company Name]
Date: June 2, 2054
Objective: To ensure comprehensive training for Clinical Research Coordinators, covering key aspects of their role and responsibilities in conducting successful clinical trials. |
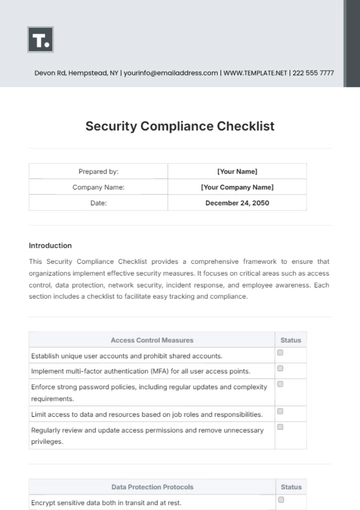
I. Regulatory Compliance and Ethics
Understand and adhere to Good Clinical Practice (GCP) guidelines.
Demonstrate knowledge of local and international regulatory requirements.
Familiarize yourself with the Declaration of Helsinki and other ethical guidelines.
Know the procedures for obtaining and maintaining Institutional Review Board (IRB) approval.
II. Protocol and Study Procedures
Thoroughly review and understand the study protocol.
Ensure proficiency in conducting informed consent processes.
Master the study procedures and follow the protocol accurately.
Implement strategies to minimize protocol deviations and violations.
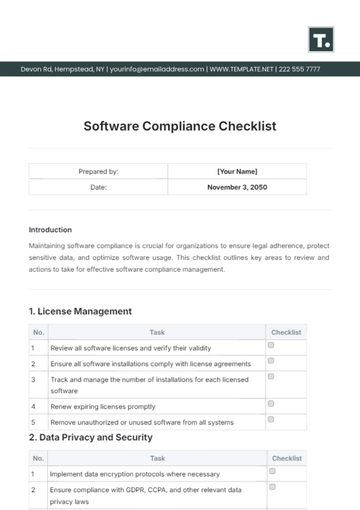
III. Data Collection and Management
Use Electronic Data Capture (EDC) systems efficiently.
Understand source document verification and data validation procedures.
Ensure accurate and timely data entry.
Familiarize yourself with adverse event reporting requirements and procedures.
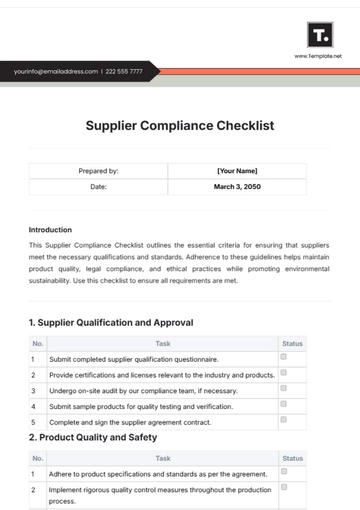
IV. Participant Interaction and Safety
Develop effective communication skills with study participants.
Implement measures to ensure participant safety and well-being.
Recognize and appropriately handle adverse events and serious adverse events.
Understand and adhere to the protocol-specific safety monitoring plan.
V. Communication and Team Collaboration
Establish effective communication channels with the study team.
Regularly update Principal Investigators on study progress.
Collaborate with other research staff and departments as needed.
Attend regular team meetings and training sessions.
- 100% Customizable, free editor
- Access 1 Million+ Templates, photo’s & graphics
- Download or share as a template
- Click and replace photos, graphics, text, backgrounds
- Resize, crop, AI write & more
- Access advanced editor
Enhance the efficiency of clinical research coordinators with the Clinical Research Coordinator Training Checklist Template from Template.net. Customizable and downloadable, this template ensures a thorough and standardized training process. Tailor the checklist seamlessly using the help of our AI Editor Tool to meet specific requirements. Download now to streamline training, improve coordination skills, and maintain excellence in clinical research endeavors.
You may also like
- Cleaning Checklist
- Daily Checklist
- Travel Checklist
- Self Care Checklist
- Risk Assessment Checklist
- Onboarding Checklist
- Quality Checklist
- Compliance Checklist
- Audit Checklist
- Registry Checklist
- HR Checklist
- Restaurant Checklist
- Checklist Layout
- Creative Checklist
- Sales Checklist
- Construction Checklist
- Task Checklist
- Professional Checklist
- Hotel Checklist
- Employee Checklist
- Moving Checklist
- Marketing Checklist
- Accounting Checklist
- Camping Checklist
- Packing Checklist
- Real Estate Checklist
- Cleaning Checklist Service
- New Employee Checklist
- Food Checklist
- Home Inspection Checklist
- Advertising Checklist
- Event Checklist
- SEO Checklist
- Assessment Checklist
- Inspection Checklist
- Baby Registry Checklist
- Induction Checklist
- Employee Training Checklist
- Medical Checklist
- Safety Checklist
- Site Checklist
- Job Checklist
- Service Checklist
- Nanny Checklist
- Building Checklist
- Work Checklist
- Office Checklist
- Training Checklist
- Website Checklist
- IT and Software Checklist
- Performance Checklist
- Project Checklist
- Startup Checklist
- Education Checklist
- Home Checklist
- School Checklist
- Maintenance Checklist
- Planning Checklist
- Manager Checklist
- Wedding Checklist
- Vehicle Checklist
- Travel Agency Checklist
- Vehicle Inspection Checklist
- Interior Design Checklist
- Backpacking Checklist
- Business Checklist
- Legal Checklist
- Nursing Home Checklist
- Weekly Checklist
- Recruitment Checklist
- Salon Checklist
- Baby Checklist
- Equipment Checklist
- Trade Show Checklist
- Party Checklist
- Hospital Bag Checklist
- Evaluation Checklist
- Agency Checklist
- First Apartment Checklist
- Hiring Checklist
- Opening Checklist
- Small Business Checklist
- Rental Checklist
- College Dorm Checklist
- New Puppy Checklist
- University Checklist
- Building Maintenance Checklist
- Work From Home Checklist
- Student Checklist
- Application Checklist