Free Facility Qualification Protocol

Prepared by: | [YOUR NAME] |
Company: | [YOUR COMPANY NAME] |
Department: | Quality Assurance (QA) |
Date: | [DATE] |
I. Objectives
The primary objective of this Facility Qualification Protocol (FQP) is to ensure the thorough assessment and verification of Elysium PharmaTech Facility to determine its suitability and functionality for its intended purpose within Pharmaceutical Manufacturing. This protocol aims to establish a systematic approach to comply with regulatory standards, mitigate risks, and maintain product quality and safety standards.
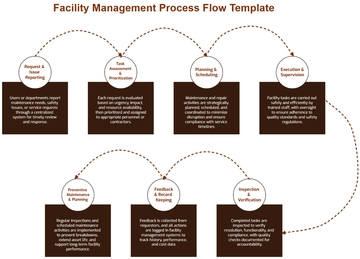
II. Protocol Overview
The FQP outlines a structured process for qualifying Elysium PharmaTech Facility, encompassing pre-qualification activities, installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). It delineates responsibilities, procedures, and documentation requirements to facilitate a comprehensive evaluation of critical systems, equipment, utilities, and processes.
III. Procedure
Pre-Qualification Activities
Review facility design documents.
Identify critical systems and equipment.
Develop a qualification plan.
Installation Qualification (IQ)
Verify equipment installation per specifications.
Calibrate instruments and sensors.
Document installation activities.
Operational Qualification (OQ)
Verify equipment functionality under operational conditions.
Conduct performance testing.
Document test results.
Performance Qualification (PQ)
Evaluate facility performance under simulated operating conditions.
Document PQ results.
IV. Data Collection
Comprehensive documentation shall be maintained throughout the qualification process, including protocols, test scripts, execution records, calibration certificates, deviation reports, and final qualification reports.
V. Safety Considerations
Adherence to safety protocols and regulations is paramount throughout the qualification process to mitigate potential risks to personnel, equipment, and the environment. Safety procedures must be strictly followed during testing and operation.
VI. Expected Results
The FQP aims to achieve the following outcomes:
Verification of facility suitability and functionality.
Compliance with regulatory requirements.
Mitigation of risks.
Maintenance of product quality and safety standards.
VII. Conclusion
The implementation of this Facility Qualification Protocol ensures a systematic and thorough evaluation of Elysium PharmaTech Facility for its intended purpose within Pharmaceutical Manufacturing. Adherence to the outlined procedures and documentation requirements is essential to achieve regulatory compliance, mitigate risks, and maintain product quality and safety standards.
- 100% Customizable, free editor
- Access 1 Million+ Templates, photo’s & graphics
- Download or share as a template
- Click and replace photos, graphics, text, backgrounds
- Resize, crop, AI write & more
- Access advanced editor
Unlock the efficiency of Facility Qualification with Template.net's editable and customizable protocol template. Crafted for seamless integration into your operations, this comprehensive resource ensures compliance and precision. Tailor-made for your specific needs, it's editable in our Ai Editor Tool, empowering you to streamline processes effortlessly. Revolutionize your facility management with this indispensable tool.