Free Process Validation Protocol

I. Introduction
The purpose of this Process Validation Protocol is to ensure that the manufacturing process consistently produces products that meet quality standards and regulatory requirements. It aims to verify process consistency, mitigate risks, and document validation activities. Additionally, it seeks to optimize processes, facilitate change control, and ensure product safety and efficacy.
II. Scope
This protocol applies to the validation of the manufacturing process for [Product Name] at [Your Company Name]. It covers all stages of production from raw material receipt to final product release.
III. Responsibilities
Quality Assurance (QA) Department: Responsible for overseeing the validation process and ensuring compliance with quality standards and regulatory requirements.
Production Department: Responsible for executing the manufacturing process according to established procedures.
Quality Control (QC) Department: Responsible for conducting in-process and final product testing to verify product quality.
Engineering Department: Responsible for providing technical support and ensuring equipment functionality.
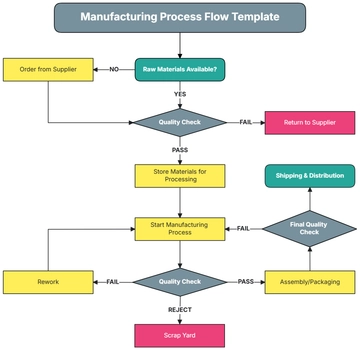
IV. Validation Protocol
Process Description:
Provide a detailed description of the manufacturing process including equipment, materials, critical process parameters, and controls.
Validation Approach:
Define validation objectives and acceptance criteria.
Identify critical process parameters and their acceptable ranges.
Determine a sampling plan for process validation.
Establish testing protocols for in-process and final product testing.
IV.3 Installation Qualification (IQ):
Verify that equipment is installed correctly and according to specifications.
Document equipment specifications, installation date, and location.
IV.4 Operational Qualification (OQ):
Verify that equipment operates within predetermined limits.
Perform equipment calibration and testing.
Document equipment performance and operational parameters.
IV.5 Performance Qualification (PQ):
Conduct process performance testing using approved protocols.
Monitor critical process parameters and record data.
Analyze results to ensure process consistency and product quality.
IV.6 Validation Report:
Compile validation results and observations.
Document deviations and corrective actions are taken.
Obtain approval from relevant stakeholders.
V. Change Control
Any changes to the manufacturing process or equipment must be evaluated for their impact on product quality and regulatory compliance. Changes require appropriate documentation, risk assessment, and validation before implementation.
VI. Documentation
All documentation related to process validation, including protocols, reports, and records, must be maintained according to established procedures. Documentation should be readily available for review by regulatory authorities.
VII. Training
Personnel involved in the manufacturing process must receive training on validation procedures, GMP (Good Manufacturing Practices), and relevant quality standards.
VIII. Conclusion
This Process Validation Protocol outlines the procedures for validating the manufacturing process of [Product Name]. By following this protocol, we ensure product quality, regulatory compliance, and continuous process improvement. Validation activities will be conducted according to established timelines and documented accordingly.
IX. Approval
This Process Validation Protocol is approved by:

[Your Name]
[Date Signed]

[Quality Assurance Name]
[Date Signed]
Protocol Templates @ Template.net
- 100% Customizable, free editor
- Access 1 Million+ Templates, photo’s & graphics
- Download or share as a template
- Click and replace photos, graphics, text, backgrounds
- Resize, crop, AI write & more
- Access advanced editor
Unlock efficiency and compliance with our Process Validation Protocol Template, offered by Template.net. This customizable, downloadable, and printable template streamlines your validation process effortlessly. Crafted with precision, it's editable in our AI Editor Tool, ensuring seamless adaptation to your specific requirements. Elevate your workflow and ensure regulatory adherence with this essential tool.