Free Audit Checklist for Medical Device Facility
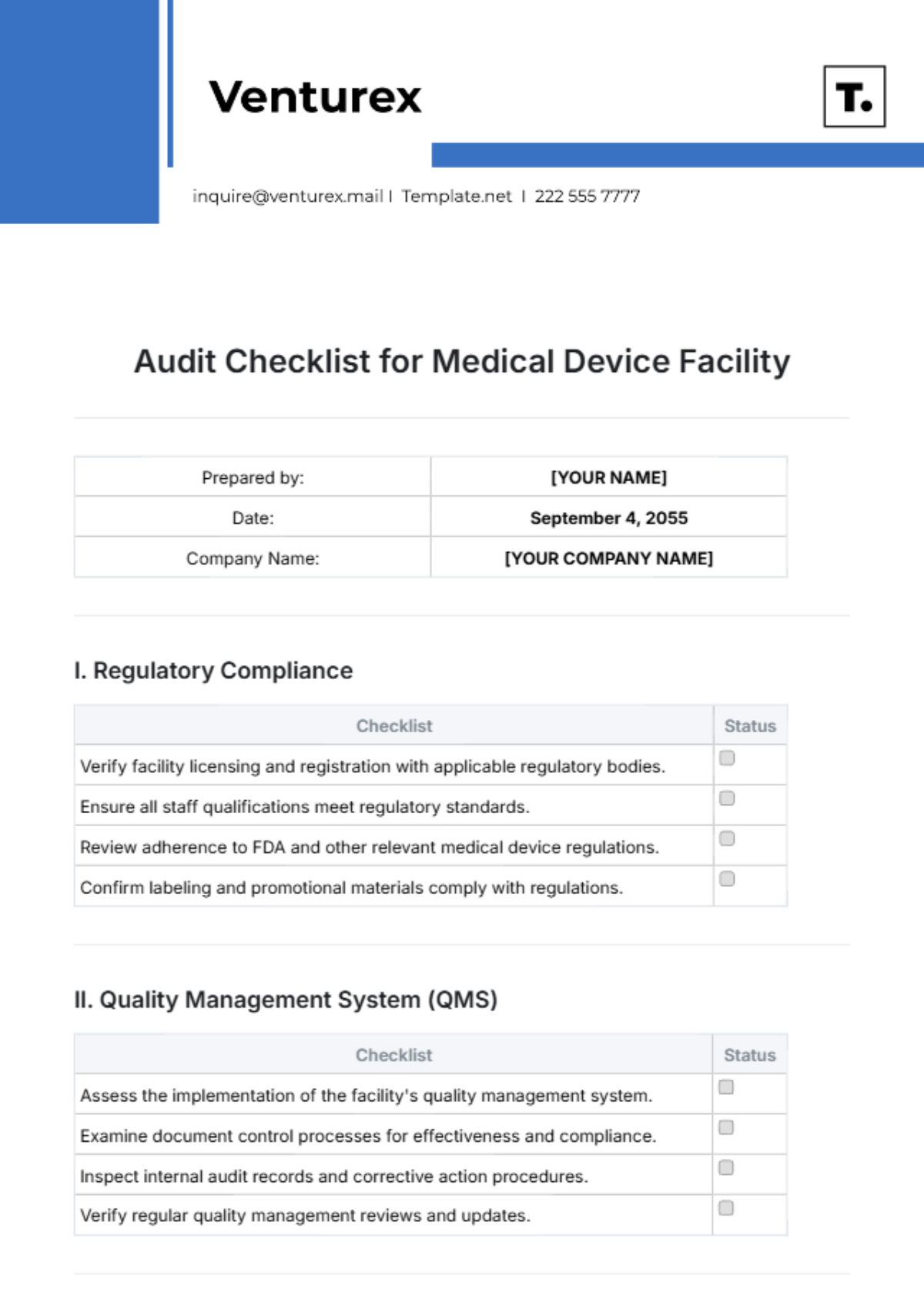
Prepared by: | [YOUR NAME] |
Date: | September 4, 2055 |
Company Name: | [YOUR COMPANY NAME] |
I. Regulatory Compliance
Checklist | Status |
|---|---|
Verify facility licensing and registration with applicable regulatory bodies. | |
Ensure all staff qualifications meet regulatory standards. | |
Review adherence to FDA and other relevant medical device regulations. | |
Confirm labeling and promotional materials comply with regulations. |
II. Quality Management System (QMS)
Checklist | Status |
|---|---|
Assess the implementation of the facility's quality management system. | |
Examine document control processes for effectiveness and compliance. | |
Inspect internal audit records and corrective action procedures. | |
Verify regular quality management reviews and updates. |
III. Production and Process Controls
Checklist | Status |
|---|---|
Evaluate cleanliness and maintenance of production equipment. | |
Review validation and control of manufacturing processes. | |
Check the traceability of raw materials and components. | |
Ensure process documentation is accurate and up-to-date. |
IV. Risk Management
Checklist | Status |
|---|---|
Audit risk management plans and activities. | |
Ensure risk assessments are conducted and documented. | |
Verify implementation and monitoring of risk control measures. | |
Review emergency preparedness and response plans. |
V. Training and Competency
Checklist | Status |
|---|---|
Evaluate training programs and materials. | |
Verify staff competency and certification records. | |
Ensure training is conducted regularly and covers relevant updates. | |
Check the effectiveness of training programs through assessments. |
VI. Facility and Environmental Controls
Checklist | Status |
|---|---|
Inspect facility layout for compliance with safety standards. | |
Review environmental monitoring results and controls. | |
Assess maintenance schedules for facility equipment. | |
Verify that waste management procedures are in place and followed. |
VII. Customer Complaints and Product Quality
Checklist | Status |
|---|---|
Review customer feedback and complaint-handling procedures. | |
Audit records of product quality issues and resolutions. | |
Ensure there is a systematic approach to product recalls. | |
Verify improvements are made based on customer feedback. |
- 100% Customizable, free editor
- Access 1 Million+ Templates, photo’s & graphics
- Download or share as a template
- Click and replace photos, graphics, text, backgrounds
- Resize, crop, AI write & more
- Access advanced editor
Explore unparalleled efficiency with our top-quality Audit Checklist for Medical Device Facility Template, exclusively available on Template.net. This meticulously crafted tool is not just editable; it's fully customizable, ensuring seamless adaptation to your unique needs. Experience unparalleled ease with our Ai Editor Tool, simplifying the auditing process like never before.
You may also like
- Cleaning Checklist
- Daily Checklist
- Travel Checklist
- Self Care Checklist
- Risk Assessment Checklist
- Onboarding Checklist
- Quality Checklist
- Compliance Checklist
- Audit Checklist
- Registry Checklist
- HR Checklist
- Restaurant Checklist
- Checklist Layout
- Creative Checklist
- Sales Checklist
- Construction Checklist
- Task Checklist
- Professional Checklist
- Hotel Checklist
- Employee Checklist
- Moving Checklist
- Marketing Checklist
- Accounting Checklist
- Camping Checklist
- Packing Checklist
- Real Estate Checklist
- Cleaning Checklist Service
- New Employee Checklist
- Food Checklist
- Home Inspection Checklist
- Advertising Checklist
- Event Checklist
- SEO Checklist
- Assessment Checklist
- Inspection Checklist
- Baby Registry Checklist
- Induction Checklist
- Employee Training Checklist
- Medical Checklist
- Safety Checklist
- Site Checklist
- Job Checklist
- Service Checklist
- Nanny Checklist
- Building Checklist
- Work Checklist
- Office Checklist
- Training Checklist
- Website Checklist
- IT and Software Checklist
- Performance Checklist
- Project Checklist
- Startup Checklist
- Education Checklist
- Home Checklist
- School Checklist
- Maintenance Checklist
- Planning Checklist
- Manager Checklist
- Wedding Checklist
- Vehicle Checklist
- Travel Agency Checklist
- Vehicle Inspection Checklist
- Interior Design Checklist
- Backpacking Checklist
- Business Checklist
- Legal Checklist
- Nursing Home Checklist
- Weekly Checklist
- Recruitment Checklist
- Salon Checklist
- Baby Checklist
- Equipment Checklist
- Trade Show Checklist
- Party Checklist
- Hospital Bag Checklist
- Evaluation Checklist
- Agency Checklist
- First Apartment Checklist
- Hiring Checklist
- Opening Checklist
- Small Business Checklist
- Rental Checklist
- College Dorm Checklist
- New Puppy Checklist
- University Checklist
- Building Maintenance Checklist
- Work From Home Checklist
- Student Checklist
- Application Checklist