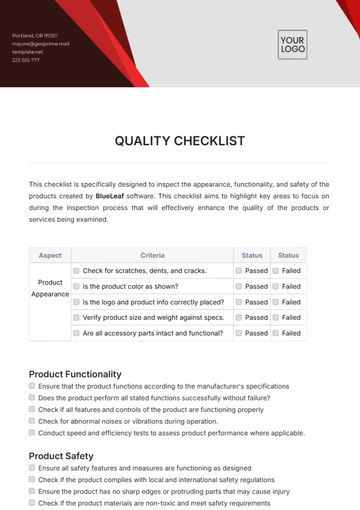
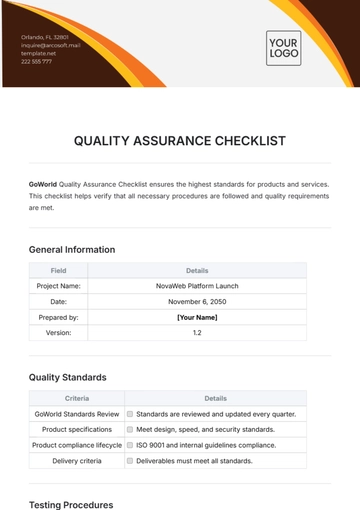
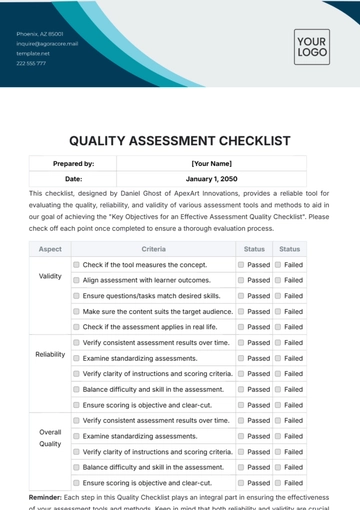
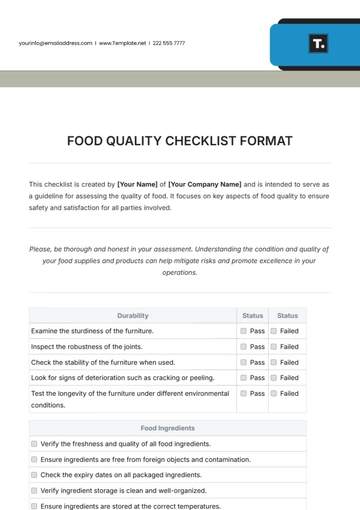
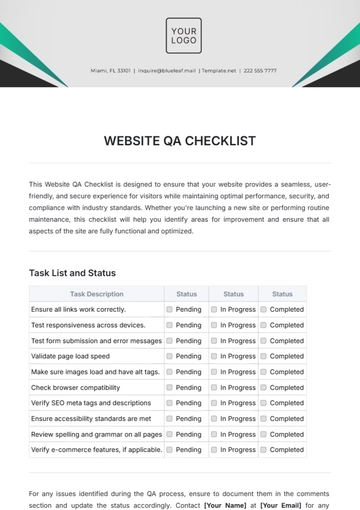
Free Quality Assurance Manager Checklist
Prepared by: [Your Name]
Company: [Your Company Name]
Date: [Date]
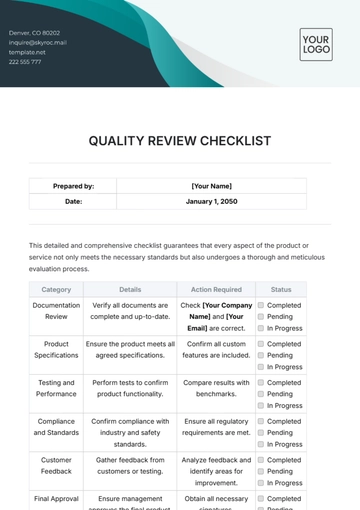
I. Document Control
Task Description | Status | Comments |
|---|---|---|
Document control review | Update required for recent changes | |
Process audit completion | Schedule audit for March 10, 2051 | |
Training program evaluation | Effective with current standards | |
Compliance requirements checklist | Awaiting external audit feedback | |
Non-conformance action closure | All corrective actions implemented | |
Initiate continuous improvement projects. | Review project proposals in the upcoming meeting. |
II. Process Evaluation
Process Evaluation | Status | Comments |
|---|---|---|
Process Mapping | All key processes were mapped and validated by November 15, 2050. | |
Process Audits | The next audit is scheduled for March 10, 2051. | |
Performance Metrics | Metrics for Q1 2051 are under analysis; findings are due by April 5, 2051. |
III. Compliance Requirements
Compliance Requirement | Description | Status | Comments |
|---|---|---|---|
Regulatory Requirements | Ensure compliance with industry regulations and standards. | Compliance audit completed as of December 20, 2050. | |
Internal Policies | Verify adherence to organizational policies and procedures. | Review of internal policies underway; completion expected by February 15, 2051. | |
External Audits | Prepare for and respond to external audit findings. | Preparing documentation for the upcoming external audit scheduled for March 25, 2051. |
IV. Training and Competence
Training and Competence | Description | Status | Comments |
|---|---|---|---|
Training Programs | Develop, implement, and evaluate training programs for staff. | The new training program launched successfully on January 5, 2051. | |
Competency Assessment | Assess employee competencies to ensure they meet job requirements. | Competency assessments are scheduled for February 10-15, 2051. | |
Training Records | Maintain accurate and up-to-date training records for all staff. | Record updates ongoing; expected completion by January 30, 2051. |
V. Non-Conformance Management
Non-Conformance Management | Description | Status | Comments |
|---|---|---|---|
Identification | Identify and document non-conformances consistently. | Non-conformance identification log updated as of December 28, 2050. | |
Corrective Actions | Implement corrective actions to address non-conformances. | All corrective actions were successfully implemented by January 10, 2051. | |
Preventive Actions | Establish preventive measures to avoid the recurrence of issues. | Preventive measures are being developed; completion is expected by February 5, 2051. |
VI. Continuous Improvement
Continuous Improvement | Description | Status | Comments |
|---|---|---|---|
Feedback Mechanisms | Implement feedback systems to gather insights from stakeholders. | Feedback system integration is scheduled for February 20, 2051. | |
Process Improvement Projects | Initiate continuous improvement projects to enhance quality. | Review project proposals in the next management meeting on March 1, 2051. | |
Review and Analysis | Regularly analyze data and trends to identify improvement opportunities. | Monthly data analysis continues; the next review is scheduled for March 5, 2051. |
Task List and Status
Task Description | Status | Comments |
|---|---|---|
Document control review |
| Update required for recent changes |
Process audit completion |
| Schedule audit for March 10, 2051 |
Training program evaluation |
| Effective with current standards |
Compliance requirements checklist |
| Awaiting external audit feedback |
Non-conformance action closure |
| All corrective actions implemented |
Initiate continuous improvement projects |
| Review project proposals in the upcoming meeting |
- 100% Customizable, free editor
- Access 1 Million+ Templates, photo’s & graphics
- Download or share as a template
- Click and replace photos, graphics, text, backgrounds
- Resize, crop, AI write & more
- Access advanced editor
Maintain high standards with the Quality Assurance Manager Checklist Template from Template.net. This editable and customizable template helps you ensure that all quality checks are completed thoroughly. Use our AI Editor Tool to adjust the checklist to fit your specific QA processes. Safeguard your products and services by promoting consistency and excellence with this vital resource.
You may also like
- Cleaning Checklist
- Daily Checklist
- Travel Checklist
- Self Care Checklist
- Risk Assessment Checklist
- Onboarding Checklist
- Quality Checklist
- Compliance Checklist
- Audit Checklist
- Registry Checklist
- HR Checklist
- Restaurant Checklist
- Checklist Layout
- Creative Checklist
- Sales Checklist
- Construction Checklist
- Task Checklist
- Professional Checklist
- Hotel Checklist
- Employee Checklist
- Moving Checklist
- Marketing Checklist
- Accounting Checklist
- Camping Checklist
- Packing Checklist
- Real Estate Checklist
- Cleaning Checklist Service
- New Employee Checklist
- Food Checklist
- Home Inspection Checklist
- Advertising Checklist
- Event Checklist
- SEO Checklist
- Assessment Checklist
- Inspection Checklist
- Baby Registry Checklist
- Induction Checklist
- Employee Training Checklist
- Medical Checklist
- Safety Checklist
- Site Checklist
- Job Checklist
- Service Checklist
- Nanny Checklist
- Building Checklist
- Work Checklist
- Office Checklist
- Training Checklist
- Website Checklist
- IT and Software Checklist
- Performance Checklist
- Project Checklist
- Startup Checklist
- Education Checklist
- Home Checklist
- School Checklist
- Maintenance Checklist
- Planning Checklist
- Manager Checklist
- Wedding Checklist
- Vehicle Checklist
- Travel Agency Checklist
- Vehicle Inspection Checklist
- Interior Design Checklist
- Backpacking Checklist
- Business Checklist
- Legal Checklist
- Nursing Home Checklist
- Weekly Checklist
- Recruitment Checklist
- Salon Checklist
- Baby Checklist
- Equipment Checklist
- Trade Show Checklist
- Party Checklist
- Hospital Bag Checklist
- Evaluation Checklist
- Agency Checklist
- First Apartment Checklist
- Hiring Checklist
- Opening Checklist
- Small Business Checklist
- Rental Checklist
- College Dorm Checklist
- New Puppy Checklist
- University Checklist
- Building Maintenance Checklist
- Work From Home Checklist
- Student Checklist
- Application Checklist