Free Production Log
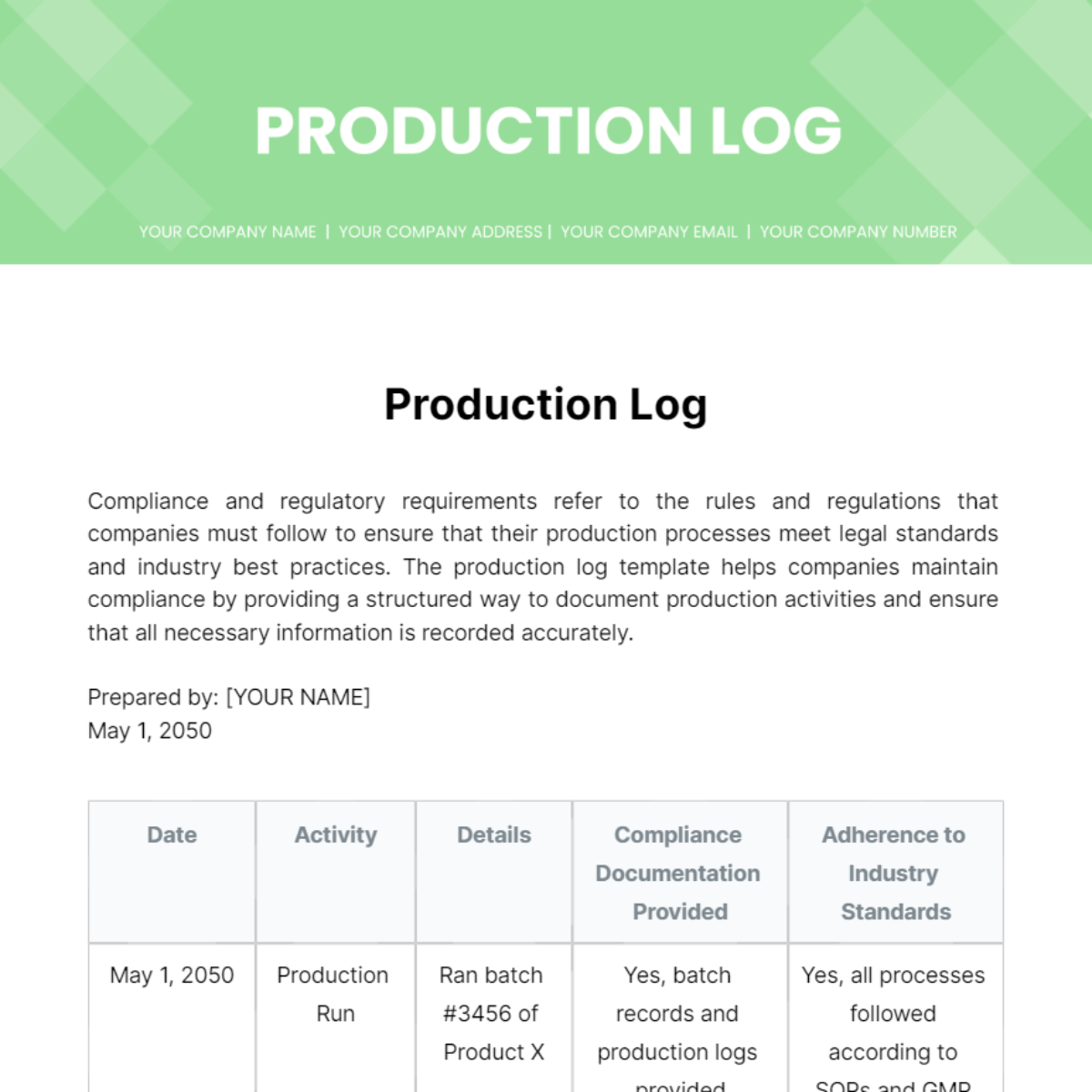
Compliance and regulatory requirements refer to the rules and regulations that companies must follow to ensure that their production processes meet legal standards and industry best practices. The production log template helps companies maintain compliance by providing a structured way to document production activities and ensure that all necessary information is recorded accurately.
Prepared by: [YOUR NAME]
May 1, 2050
Date | Activity | Details | Compliance Documentation Provided | Adherence to Industry Standards |
|---|---|---|---|---|
May 1, 2050 | Production Run | Ran batch #3456 of Product X | Yes, batch records and production logs provided | Yes, all processes followed according to SOPs and GMP guidelines |
May 1, 2050 | Quality Control Testing | Tested batch #3456 for purity and potency | Yes, the Certificate of Analysis provided | Yes, testing conducted according to USP standards |
May 1, 2050 | Packaging | Packaged batch #3456 into finished product containers | Yes, packaging records and batch documentation provided | Yes, packaging was conducted in a clean room environment following cGMP regulations |
May 1, 2050 | Labeling | Applied labels to finished product containers | Yes, labeling records and batch documentation provided | Yes, labeling is done according to FDA regulations and product specifications |
May 1, 2050 | Inventory Management | Transferred finished products to the warehouse for storage | Yes, inventory transfer records and documentation provided | Yes, inventory is managed according to FIFO principles and warehouse SOPs |
May 1, 2050 | Regulatory Audit Preparation | Reviewed production records and documentation for an upcoming audit | Yes, the audit preparation checklist and documentation provided | Yes, all necessary documents are organized and ready for audit inspection |
Notes:
The production log demonstrates a systematic approach to documenting each stage of the production process, ensuring traceability and accountability.
Compliance documentation, including batch records, Certificates of Analysis, packaging records, labeling records, and inventory transfer records, are consistently provided for each activity, indicating a strong commitment to regulatory compliance.
Adherence to industry standards, such as SOPs, GMP guidelines, USP standards, cGMP regulations, and FDA regulations, is evident throughout the production process, reflecting a dedication to quality and safety.
The proactive approach to regulatory audit preparation, with a thorough review of production records and documentation, showcases readiness for audits and emphasizes the importance of compliance in the manufacturing operation.
- 100% Customizable, free editor
- Access 1 Million+ Templates, photo’s & graphics
- Download or share as a template
- Click and replace photos, graphics, text, backgrounds
- Resize, crop, AI write & more
- Access advanced editor
Introducing the Production Log Template from Template.net. Streamline your workflow with this editable and customizable tool. Keep track of every detail effortlessly as you manage your projects seamlessly. Whether it's tracking progress or logging milestones, this template, editable in our Ai Editor Tool, ensures efficiency and organization every step of the way.