Free Clean Room Validation Protocol

Name: | [Your Name] |
Company: | [Your Company Name] |
Department: | [Your Department] |
Date: | [Date] |
I. Introduction:
This document serves as a comprehensive guideline for the validation of clean room facilities within [Your Company Name]. Clean rooms play a critical role in industries such as pharmaceuticals and aerospace, necessitating stringent adherence to cleanliness standards to ensure product quality and safety.
II. Objective(s):
The primary objectives of this protocol are to establish procedures for validating the cleanliness and integrity of clean room facilities, ensure compliance with regulatory standards, mitigate risks associated with contamination, optimize operational processes, and facilitate continuous improvement efforts.
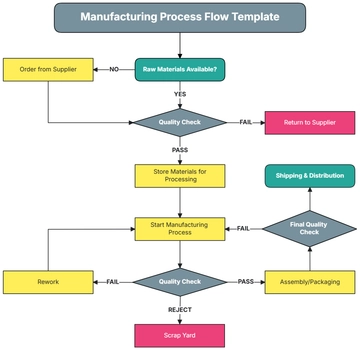
III. Protocol Overview:
The Clean Room Validation Protocol outlines a systematic approach to assessing the cleanliness and integrity of clean room facilities. It encompasses procedures for evaluating various parameters such as air quality, surface cleanliness, and particle counts to ensure the reliability and consistency of clean room operations.
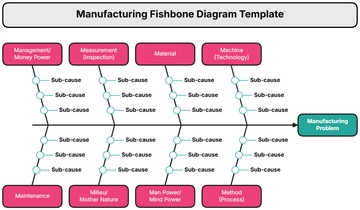
IV. Materials and Equipment:
Particle counters
Airflow measurement devices
Surface sampling kits
Microbiological monitoring equipment
Calibration standards
Clean room garments and personal protective equipment
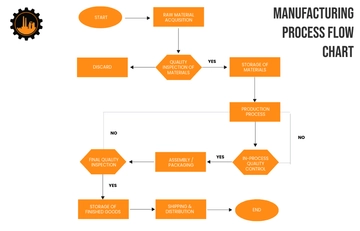
V. Methods/Procedure:
Preparing the clean room for validation.
Conducting pre-validation checks and environmental monitoring.
Performing particle count measurements.
Assessing air velocity and airflow patterns.
Conducting surface cleanliness testing.
Sampling for microbiological contamination, if applicable.
Analyzing collected data and comparing results against acceptance criteria.
Documenting findings and deviations.
Implementing corrective actions if necessary.
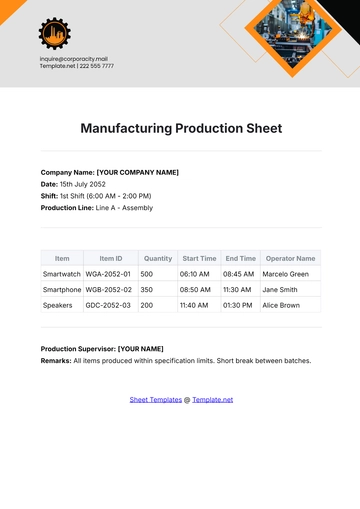
VI. Data Collection:
Data collected during the clean room validation process shall include:
Particle counts at various locations
Air velocity measurements
Surface cleanliness test results
Microbiological sampling data (if applicable)
Any deviations from the acceptance criteria
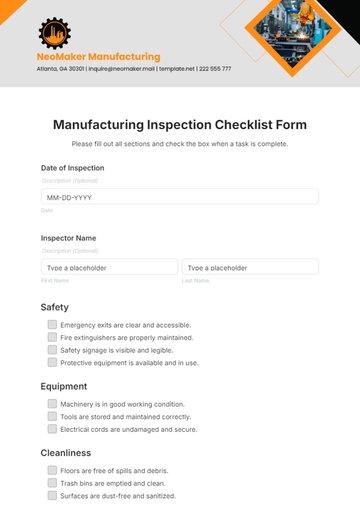
VII. Safety Considerations:
Adhere to personal protective equipment (PPE) requirements.
Handle testing equipment and chemicals according to safety guidelines.
Ensure proper ventilation and air quality within the clean room during validation activities.
Follow established procedures for waste disposal.
VIII. Expected Results:
The expected results of the clean room validation process include:
Compliance with regulatory cleanliness standards.
Demonstration of the clean room's suitability for its intended purpose.
Identification of any deviations or areas for improvement.
Documentation of validation findings and corrective actions.
IX. Conclusion:
In conclusion, the Clean Room Validation Protocol provides a systematic framework for ensuring the cleanliness and integrity of clean room facilities. By adhering to the procedures outlined in this protocol, [Your Company Name] can uphold quality assurance standards, mitigate contamination risks, and optimize cleanroom operations.
- 100% Customizable, free editor
- Access 1 Million+ Templates, photo’s & graphics
- Download or share as a template
- Click and replace photos, graphics, text, backgrounds
- Resize, crop, AI write & more
- Access advanced editor
Enhance your cleanroom validation process effortlessly with the Clean Room Validation Protocol Template from Template.net. Our meticulously crafted template, offered by Template.net, streamlines the validation process with its customizable and downloadable features. Crafted for convenience, this template is editable in our AI Editor Tool, ensuring seamless customization to fit your unique requirements.